Molecular Functionalities of Protein Sectors 4
Molecular Functionalities of Protein Sectors 4
Pau Creixell & Michael B. Yaffe, with thanks to Lucia Suarez Lopez for artistic direction
MIT Department of Biology, MIT Department of Biological Engineering, Koch Institute at MIT
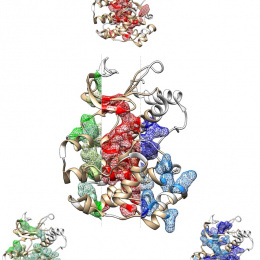
Our genomes encode thousands of proteins with multiple protein domains. Even though each protein domain is thought to perform a specific molecular function, how single protein domains such as the kinase domain (depicted in the image) are capable of performing multiple, complex functions has remained enigmatic. Using a novel computational method, named comparative coupling analysis, three distinct sets of residues (or as we call them protein sectors) performing different molecular functionalities were identified. The residues illustrated in red include the catalytic core of the kinase domain, the residues in green involve substrate specificity and residues in blue connect to sites of allosteric regulation more distant to the active site. I wanted to analyze the structural positioning of the residues forming the three different sets of residues (protein sectors) when superimposed on the structure of the kinase domain. As part of the analysis we not only considered the three protein sectors separately, but also in relation to one another.